Important Notes
Cryoalfa devices are reusable instruments intended for the controlled treatment of benign skin lesions through the application of extreme cold using nitrous oxide (N₂O), supplied in disposable 16 g or 25 g cartridges. The main indications include, but are not limited to, warts, actinic and seborrheic keratoses, granulomas, dermatofibromas, condylomas, molluscum contagiosum, lentigines, and angiofibromas.
These devices are primarily used in dermatology but may also be employed by other healthcare professionals, including general practitioners, pediatricians, gynecologists, urologists, surgeons, podiatrists, and dentists. Use is restricted to trained professionals and must comply with applicable national regulations.
Instructions for Use
Insertion of the N₂O Cartridge (Super & Lux)
Only original Cryoalfa cartridges must be used. Unscrew the protective cap from the cartridge and remove the valve protection cap. Screw the cartridge clockwise into the device thread until slight resistance is felt. Tighten carefully and do not overtighten.
The cartridge is equipped with a valve that allows connection and disconnection without gas leakage. Empty cartridges must be replaced with new ones.
Functional Test
A functional test must be performed before each use. To check the flow of liquid gas, place the glass capillary directly onto a grey cardboard surface and press the lever for one second. The resulting liquid spot must have a minimum diameter of 5–6 mm. Do not use defective devices under any circumstances.
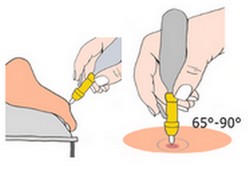
Liquid Freeze Treatment
Remove the protective cap from the glass tip, position the device over the lesion at an angle between 65° and 90°, and apply the N₂O by pressing the lever.
Contact Freeze Treatment
Pressing the lever initiates cooling of the contact applicator. After approximately 15 seconds, the applicator reaches its operating temperature (-50 °C). The cooled applicator is then applied directly to the lesion using controlled pressure.
Treatment Duration
The treatment duration depends on the depth of the lesion. Detailed information is provided in the section dedicated to treatment times.
Cleaning
The device must be cleaned as soon as possible after use. Components that come into contact with the patient must be cleaned and disinfected no earlier than 10 minutes after use. Remove the gas cartridge and eliminate any visible contamination using a disposable wipe or paper towel.
Manual Cleaning
Prepare the cleaning solution according to the manufacturer’s instructions (e.g. Bomix 1%). Immerse the applicator, ensuring that the entire surface is fully wetted. Allow the solution to act for at least 5 minutes, then rinse with potable-quality water for a minimum of 1 minute. Repeat the procedure if necessary.
Disinfection
Prepare the disinfectant solution in accordance with the manufacturer’s recommendations (e.g. Bomix Plus 2%). Immerse the applicator for the specified contact time (e.g. 5 minutes). Rinse twice with fully demineralized water for at least 1 minute each. Dry using a lint-free disposable cloth or medical compressed air.
Sterilization in a Sterilizer
Use a sterilizer compliant with DIN EN 285 or DIN EN 13060 (Type B process). Perform steam sterilization with fractional pre-vacuum at 134 °C for a minimum of 3 minutes or at 132 °C for a minimum of 3 minutes. After completion of the cycle, remove the devices and allow them to cool.
Storage
When not in use, store the device in its original packaging, ensuring that the lever cannot be accidentally activated. Protect gas cartridges from heat and direct sunlight. Never expose cartridges to temperatures exceeding +50 °C. Store cartridges at an ambient temperature of approximately 21 °C, with the tip protected by the cap.
Disposal
Disposal must be carried out in accordance with applicable local regulations. Empty gas cartridges may be disposed of as metal waste.
Safety Instructions
The device must be used exclusively in accordance with these instructions. Any modification is prohibited and will void the warranty and the manufacturer’s liability.
Gas cartridges are under high pressure and must be handled in strict compliance with safety instructions. Never use a damaged device. Any device that has been dropped must be inspected by the manufacturer before reuse. When installing a cartridge, do not apply force and ensure proper alignment before tightening.
Example of Wart Treatment
Outline and measure the lesion, then record the data in the patient’s file. Debride the wart close to the point of bleeding; in the event of bleeding, particularly for plantar warts, use a hemostatic solution.
Position the patient so that the lesion is facing upward. Apply the device directly to the lesion at an angle between 65° and 90°, and activate the lever. The freezing duration depends on the type and depth of the lesion (approximately 1 mm penetration per 3 seconds).
Freezing begins immediately and may cause an intense cold sensation or moderate pain. Redness typically appears within a few minutes after treatment. A small area of surrounding healthy skin may also be affected.
For plantar treatments, apply an adhesive dressing after the procedure. A follow-up examination is recommended within two weeks; multiple treatment sessions may be required.
Warranty
The warranty is limited exclusively to the replacement of defective parts. No warranty claims shall be accepted for damage resulting from improper use, storage, or transport, or from failure to comply with the provided instructions.
The manufacturer’s warranty and liability are also excluded in cases of loss of working time, improper handling, failure to perform treatment or resulting consequences, as well as non-compliance with safety instructions.
Disclaimer
Improper use of the device, including freezing that is more intense or of longer duration than recommended, may result in injury to the patient and/or the operator.
Cryoalfa Europe GmbH, together with its affiliated companies, directors, employees, agents, contractors, and shareholders, shall not be liable for any death, physical or psychological injury, loss, or damage arising directly or indirectly from the use, possession, condition, or specifications of Cryoalfa products. This limitation of liability applies regardless of the legal basis invoked, including contractual liability, tort, strict liability, breach of warranty, or failure to meet an essential obligation.
This limitation also applies where damage results from errors of judgment by Cryoalfa Europe GmbH or its representatives, even if the possibility of such damage was known or foreseeable.
By using Cryoalfa products, the user expressly waives any claims against Cryoalfa Europe GmbH and the aforementioned parties for any direct or indirect, incidental, consequential, special, or punitive damages, as well as for all related costs, expenses, penalties, or legal proceedings, including legal defense fees.
